SafeView™ Classic
Specifications
| SKU | G108 |
| Name | SafeView™ Classic |
| Unit | 1.0 ml |
| LED Viewer Compatibility | Yes |
| Description |
abm’s SafeView™ Classic is a safe, improved formulation of nucleic acid stain for agarose gel electrophoresis. SafeView™ Classic directly replaces toxic Ethidium Bromide and eliminates its associated contamination risks with glassware, gel apparatus and environment. Features: |
| Applications |
Safe Detection of dsDNA, ssDNA and RNA in agarose gels. |
| Concentration | 10,000X |
| Format | Pre-cast or Post-stain |
| Storage Condition |
Store at 18-25°C |
| Note |
All SafeView DNA Stains are ISO-13485 certified. |
Documents
Other
FAQs
| Can SafeView Classic be a replacement for ethidium bromide? Can I do gel extraction with it? | |
|
SafeView Classic can be used as a complete replacement for your ethidium bromide workflow. Yes, SafeView Classic can be used during gel extraction - in house testing demonstrated that gel extraction of DNA fragments using a SafeView Classic gel resulted in no decrease in ligation efficiency vs ethidium bromide. |
| How does SafeView work and why is it not carcinogenic? | |
|
abm's SafeView products contain fluorescent compounds that have a strong affinity to DNA and RNA nucleic acids. Once bound to nucleic acids, the compound fluoresces under specific wavelength of light which can then be visualized using a standard UV/Blue light imager. There may be some unknown effects of SafeView products that have not been documented in literature but these would also apply to popular SYBRSafe as well; however, SafeView products are not as carcinogenic as ethidium bromide. |
| How do I use SafeView products? | |
|
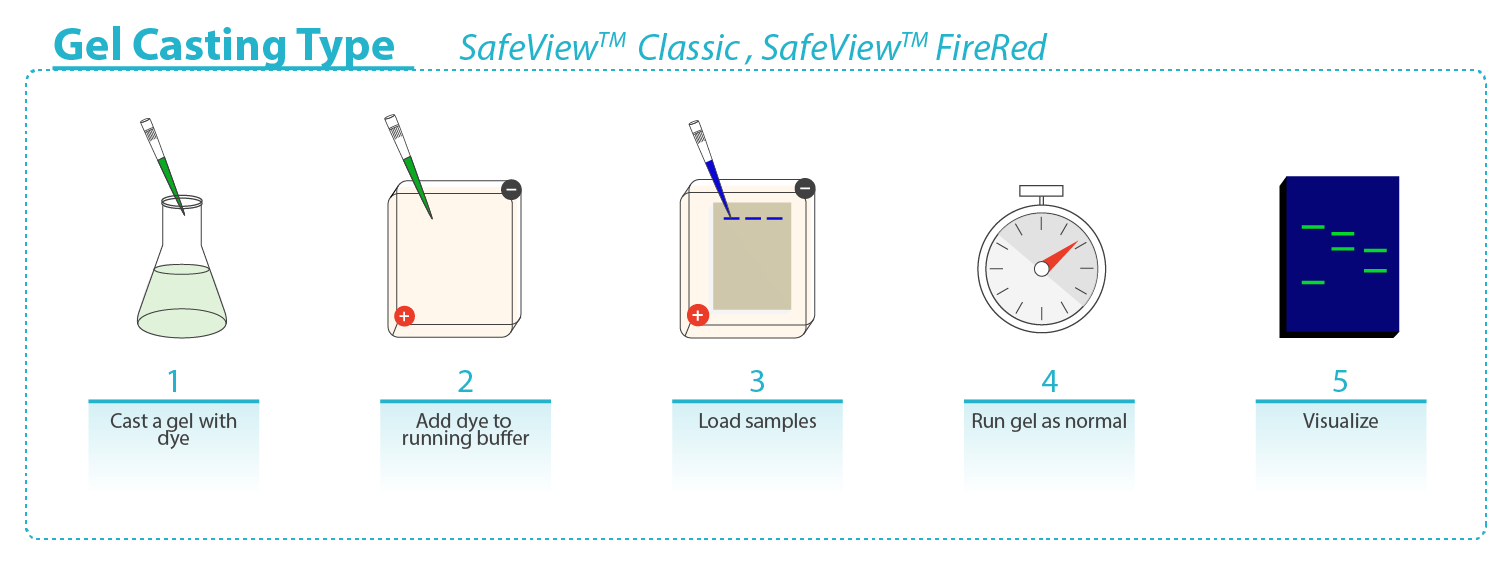
Safe-Red and Safe-Green are in a 6X loading dye format: mix samples and DNA marker with Safe-Red or Safe-Green at a 1:5 (dye : sample) dilution ratio, and load onto an unstained gel. SafeView Classic and Safe-Red Gel are in a 10,000X format: for pre-cast, add 10µl SafeView Classic or Safe-Red Gel per 100 ml molten agarose, mix gently and cast the gel; for post-stain, prepare and run an unstained agarose gel, next submerge the gel in post-staining solution of 30 μl SafeView Classic or Safe-Red Gel per 100 ml 1X TAE or 1X TBE buffer for 30min in the dark with light agitation, image accordingly. |
| Can the SafeView products differentiate double stranded vs single stranded nucleic acids? | |
|
No, our SafeView products will bind to both double-stranded and single-stranded nucleic acids, albeit with lesser efficiency for single-stranded nucleic acid species.
|
| At what temperature do I store the SafeView products? | |
|
SafeView Classic, Safe-Red and Safe-Red Gel should be stored at 18-25°C. Safe-Green should be stored at 4°C. |
| Do I need a special filter for photography of DNA gels stained with SafeView products? | |
|
All SafeView products are compatible with UV or Blue Light imagers. However, Safe-Green and SafeView Classic are more optimally visualized under Blue Light, making these stains safer for the user and their samples.
|
| How long does SafeView Classic stain last in a gel? | |
|
Our in-house testing has shown that SafeView Classic stained gels (>10ng DNA loaded per lane) can still be effectively visualized up to 1 week later with only a slight decrease in brightness. Gels should be stored properly to maintain a good signal, sealed in a plastic bag with wet paper towel loosely wrapped around the gel, away from light. Similarly, a pre-cast unused SafeView Classic gel can be stored and used up to 1 week after being prepared provided they are stored in ideal conditions (sealed in plastic bag, away from light).
|
| What is the sensitivity of the SafeView products? | |
|
Safe-Green: 0.2-0.6 ng DNA per band Safe-Red: 0.6-1.0 ng DNA per band Safe-Red Gel: 0.6-1.0 ng DNA per band SafeView Classic: 1.0-2.0 ng DNA per band |
| Can SafeView products be used to post-stain gels? | |
|
Yes, SafeView Classic and Safe-Red Gel can both be used for either pre-cast or post-stain gels.
|
| Why is the EtBr signal stronger in the pictures when I compare SafeView with EtBr? | |
|
Most gel imaging systems have been optimized for EtBr so this is a likely reason why the EtBr signal may be stronger in the pictures.
|
| Is there any guidance on how long the gel lasts once the stain has been added? | |
|
A pre-cast unused SafeView Classic gel can be stored and used up to 1 week after being prepared provided they are stored in ideal conditions (sealed in plastic bag, away from light). If it is stored longer than a week, there will be decreased resolution.
|
| How do I dispose Agarose Gels stained with Safeview and how do I dispose buffers and gloves? | |
|
Dispose SafeView™ Classic as you would any other non-carcinogenic fluorescent dye (eg. Acridine orange; Propidium iodide).
All gels and contaminated “non-sharp” lab debris (e.g., gloves, pads, towels, tubes, etc.) that are processed using this dye can be discarded in the trash.
Spent running buffer solutions and destaining solutions that contain the dyes can either be collected and disposed of through the HWMU or collected and run through an approved filter device.
The buffer solutions that have been run through the approved filter should be checked under the appropriate light source for complete removal of the dyes, and if it passes (does not fluoresce), the liquid can be disposed of down the drain with a copious amount of water as long as no other materials are present that would cause the material to be a Hazardous Waste.
The filters that have been used up and are no longer effective must be disposed of through the HWMU.
|
| Will I need an additional loading buffer for my samples? | |
|
If using Safe-Green or Safe-Red, you will not need to add any additional loading dyes or buffers. If using SafeView Classic or Safe-Red Gel, you will need to add an appropriate DNA loading dye to your samples in order for samples to properly settle and stay in the wells during electrophoresis. |
| Do SafeView products give problems in the process of cloning? | |
|
We recommend using SafeView Classic or Safe-Red Gel which can both be used for the gel extraction of DNA fragments destined for cloning. We have observed no decrease in cloning efficiency using these stains.
|
| We see migrations and band shifting of our fragments when using Safe-Green. Are there any recommendations that you can give us to minimize this band shifting? | |
|
Band shifting to a certain degree is unavoidable for any nucleic acid stain as most of these fluorescent compounds are large, positively charged molecules. Loading dye format nucleic acid stains, while convenient, may also suffer from more prominent band shifting due to the stain binding and migrating with the sample simultaneously during electrophoresis. Post-staining, while takes longer to complete, may be the optimal choice of nucleic acid stain strategy if band shifting issue is a priority. |
| I cannot see 100bp and 200bp bands on a 1% gel. What should I do? | |
|
It is often difficult to detect smaller 100bp and 200bp bands on a 1% gel especially if samples are of low concentration. We recommend visualizing smaller fragments on a higher concentration gel - ideally 2% agarose.
|
| Can I use SafeView products in polyacrylamide gels? | |
|
SafeView products are designed for use with agarose gels, and are not optimized for polyacrylamide gels.
|
| What device do you use to see the electrophoresis bands? | |
|
We use both UV, Blue light and LED transilluminators. Our gel QC pictures are taken under UV illumination.
|
| Will staining appear the same when using UV light and LED (same colors)? | |
|
UV and LED will give the same colors.
|
| Which of the SafeView products will work with blue light / LED? | |
|
All of our SafeView products are compatible with UV and blue light.
|
| Can SafeView products be stored at -20°C? | |
|
No, please store SafeView Classic, Safe-Red and Safe-Red Gel at 18-25°C. Safe-Green should be stored at 4°C.
|
| Can SafeView Classic penetrate cell membranes? | |
|
Yes, SafeView Classic can penetrate living cell membranes.
|
| What color is the tracking dye during electrophoresis? | |
|
Safe-Green and Safe-Red contain a blue tracking dye which will migrate at ~300bp after electrophoresis.
|
| Can you describe what particular imaging system that one should use to image the gel? | |
|
Any imaging system with UV, Blue light and/or LED should work.
|
References
- Ish-Shalom, S et al. "Analysis of fungal gene expression by Real Time quantitative PCR" Methods Mol Biol. 638:103-104 (2010). DOI: 10.1007/978-1-60761-611-5_7. Application: PCR Products Viewing.
- Pyo, JS et al. "Activation of nuclear factor-κB contributes to growth and aggressiveness of papillary thyroid carcinoma" Pathol Res Pract. 209 (4):228-232 (2013). DOI: 10.1016/j.prp.2013.02.004. Application: PCR Products viewing.
- Diza-Balzac, CA et al. "Calbindin-D32k Is Localized to a Subpopulation of Neurons in the Nervous System of the Sea Cucumber Holothuria glaberrima (Echinodermata)" PLoS 7 (3):e32689 (2012). DOI: 10.1371/journal.pone.0032689. Application: PCR Products Viewing.
- Kim, NH et al. "Reactive oxygen species regulate context-dependent inhibition of NFAT5 target genes" Exp Mol Med 45:e32 (2013). DOI: 10.1038/emm.2013.61. Application: PCR Products Viewing.
- Wang, AY et al. "Key functional regions in the histone variant H2A. Z C-terminal docking domain" Mol. Cell. Biol. 31(18):3871-3884 (2011). DOI: 10.1128/MCB.05182-11.
- Ibeagha-Awemu, EM et al. "Proteomics, genomics, and pathway analyses of Escherichia coli and Staphylococcus aureus infected milk whey reveal molecular pathways and networks involved in mastitis" J. Proteome Res. 9 (9):4604-4619 (2010). DOI: 10.1021/pr100336e. PubMed: 20704270. Application: PCR Products Viewing.
- Machida, RJ et al. "PCR primers for metazoan nuclear 18S and 28S ribosomal DNA sequences" PLoS ONE 7 (9):e46180 (2012). DOI: 10.1371/journal.pone.0046180. PubMed: 23049971. Application: PCR Products Viewing.
- Goo, BG et al. "Bacillus thuringiensis: a specific gamma-cyclodextrin producer strain" Carbohydr. Res. 07-Dec:386 (2014). DOI: 10.1016/j.carres.2013.12.005. PubMed: 24456970. Application: PCR Products Viewing.
- Lam, SW et al. "Rapid, specific and quantitative polymerase chain reaction (PCR) detection of pathogenic protozoa Entamoeba histolytica for drinking water supply" Water Science & Technology: Water Supply 11 (4):418-425 (2011). DOI: 10.2166/ws.2011.057. PubMed: 67657423. Application: PCR Products Viewing.
- Lee, SB et al. "Analysis of zebrafish (Danio rerio) behavior in response to bacterial infection using a self-organizing map" BMC Vet Res 7: 269 (2015). DOI: 10.1186/s12917-015-0579-2. PubMed: 26497220. Application: PCR Products Viewing.
- Keskin, A et al. "Distributions of CYP19, ERα and PGR Allele Frequencies between Fertile and Subfertile Holstein-Friesian Heifers" Kafkas Univ Vet Fak Derg 6:893-898 (2015). DOI: 10.9775/kvfd.2015.13827. Application: PCR Products Viewing.
- Mo, J et al. "Early growth response 1 (Egr-1) directly regulates GABAA receptor α2, α4, and θ subunits in the hippocampus" J Neurochem 133(4):489-500 (2015). DOI: 10.1111/jnc.13077. PubMed: 25708312. Application: PCR Products Viewing.
- Chen, T et al. "Stimulation of Proliferation and Migration of Mouse Macrophages by Type B CpG-ODNs Is F-Spondin and IL-1Ra Dependent" PLoS One 10(6):e0128926 (2015). DOI: 10.1371/journal.pone.0128926. PubMed: 26042735. Application: PCR Products Viewing.
- Hsieh, C et al. "Gallic acid exhibits risks of inducing muscular hemorrhagic liposis and cerebral hemorrhage--its action mechanism and preventive strategy" Phytother Res 29(2):267-80 (2015). DOI: 10.1002/ptr.5249. PubMed: 25403162. Application: PCR Products Viewing.
- Do, SI et al. "Decreased expression of p27 is associated with malignant transformation and extrathyroidal extension in papillary thyroid carcinoma" Tumour Biology 37.3:3359 (2016). DOI: 10.1007/s13277-015-4163-y. Application: Visualization.
- Nanduri, V et al. "Histochemical and Molecular Characterization of Spongiosal Cells in Native Tissue, Two-and Three-Dimensional Cultures of Rat Aortic Valve" J. Histology 2016:1 (2016). DOI: dx.doi.org/10.1155/2016/7680701.
- Anjali, N et al. "Intraspecific variations in cardamom (Elettaria cardamomum Maton): assessment of genomic diversity by flow cytometry, cytological studies and ISSR analysis" Springerplus 5(1):1560 (2016). DOI: 10.1186/s40064-016-3226-x.
- Dudek, M et al. "The chondrocyte clock gene Bmal1 controls cartilage homeostasis and integrity" J. Clin. Invest. 126(1):365-376 (2016). DOI: doi: 10.1172/JCI82755.
- Vivero, RJ et al. "Structural differences in gut bacteria communities in developmental stages of natural populations of Lutzomyia evansi from Colombia's Caribbean coast" Parasit. Vectors 126:365 (2016). DOI: doi: 10.1172/JCI82755.
- Zu, Y et al. "Facile and phase-defined determination of HLA alleles with morpholino-functionalized nanoparticle probes" Nanomedicine : (2016). DOI: dx.doi.org/10.1016/j.nano.2016.09.009.
- Mizan, MF et al. "Variability in biofilm formation correlates with hydrophobicity and quorum sensing among Vibrio parahaemolyticus isolates from food contact surfaces and the distribution of the genes involved in biofilm formation" Biofouling 32 (4):497-509 (2016). DOI: dx.doi.org/10.1080/08927014.2016.1149571.
- Zhao, X et al. "Optical fiber sensor based on surface plasmon resonance for rapid detection of avian influenza virus subtype H6: Initial studies." J Virol Methods 233:15-22 (2016). DOI: dx.doi.org/10.1016/j.jviromet.2016.03.007.
- Doh, EJ et al. "Identification and monitoring of Korean medicines derived from Cinnamomum spp. by using ITS and DNA marker" Genes Genomics 39(1):101-109 (2016). DOI: 10.1007/s13258-016-0476-5.
- Do, SI et al. "Decreased expression of p27 is associated with malignant transformation and extrathyroidal extension in papillary thyroid carcinoma" Tumour Biol. 37(3):3359-3364 (2016). DOI: 10.1007/s13277-015-4163-y.
- Ashrafudoulla, M., Mizan, M. F. R., Park, H., Byun, K. H., Lee, N., Park, S. H., & Ha, S. D. "Genetic Relationship, Virulence Factors, Drug Resistance Profile and Biofilm Formation Ability of Vibrio parahaemolyticus Isolated From Mussel" Frontiers in Microbiology 10: (2019). DOI: 10.3389/fmicb.2019.00513.
- Busuttil, F.. "Combining tissue engineering and gene delivery to enhance peripheral nerve regeneration (Doctoral dissertation, UCL)" University College London London:England (2019).
- Cilvez, P., & Turkyilmaz, S. "Molecular Diagnosis of Candida Species Isolated from Cases of Subclinical Bovine Mastitis" Israel Journal of Veterinary Medicine 73(3):134–140. Retrieved from (2019).
- Coban, A., Pennone, V., Sudagidan, M., Molva, C., Jordan, K., & Aydin, A. "Prevalence, virulence characterization, and genetic relatedness of Listeria monocytogenes isolated from chicken retail points and poultry slaughterhouses in Turkey" Brazilian Journal of Microbiology 50(4):1063-1073 (2019).
- Ho, Y. S., Wu, J. Y., & Chang, C. Y. "A New Natural Antioxidant Biomaterial from Cinnamomum osmophloeum Kanehira Leaves Represses Melanogenesis and Protects against DNA Damage" Antioxidants 8(10):474 (2019).
- Jeong, H.-P., Cha, J., Park, S. Y., Yoon, I. S., Lee, J. S., Heu, M. S., & Kim, J.-S. "원료 등급에 따른 명란의 위생학적 특성" 한국수산과학회지 52(4):334–343 (2019). DOI: 10.5657/KFAS.2019.0334.
- Işık, R., & Bilgen, G. "Associations between genetic variants of the POU1F1 gene and production traits in Saanen goats" Archives Animal Breeding 62(1):249–255 (2019). DOI: 10.5194/aab-62-249-2019.
- Jo, Y. D., Lee, H. Y., Ro, N. Y., Kim, S. H., Kim, J. B., Kang, B. C., & Kang, S. Y. "Mitotypes based on structural variation of mitochondrial genomes imply relationships with morphological phenotypes and cytoplasmic male sterility in peppers" Frontiers in Plant Science 10:1343 (2019).
- Keskin, D., Başbülbül, G., & Bozdoğan, B. "The effects of hypochlorous acid on microflora of chicken meat" European Journal of Biotechnology and Bioscience 7(3):41–46. Retrieved from (2019).
- Lee, Y. H., Kim, D. Y., Jeong, S. H., & Hwang, Y. J. "Effect of exposure to Asian sand dust-Particulate matter on liver Tenascin-C expression in human cancer cell and mouse hepatic tissue" The Journal of toxicological sciences 44(9):633-641 (2019).
- Lee, Y. H., Kim, D.Y., Jeong, S.H., Hwang, Y.J. "Effect of exposure to Asian sand dust-Particulate matter on liver Tenascin-C expression in human cancer cell and mouse hepatic tissue" J. Toxicol. Sci. 44(9) 633-641: (2019).
- Pereira, B. A., Zangeronimo, M. G., Castillo-Martín, M., Gadani, B., Chaves, B. R., Rodríguez-Gil, J. E., … Yeste, M. "Supplementing Maturation Medium With Insulin Growth Factor I and Vitrification-Warming Solutions With Reduced Glutathione Enhances Survival Rates and Development Ability of in vitro Matured Vitrified-Warmed Pig Oocytes" Frontiers in Physiology 9: (2019). DOI: 10.3389/fphys.2018.01894.
- Xuan, T., Anh, T., Tran, H.-D., Khanh, T., & Dat, T. "Mutation Breeding of a N-methyl-N-nitrosourea (MNU)-Induced Rice (Oryza sativa L" ssp. Indica) Population for the Yield Attributing Traits. Sustainability 11(4):1062 (2019). DOI: 10.3390/su11041062.
- 정효빈., 차장우., 박선영., 윤인성., 이정석., 허민수., & 김진수. "원료 등급에 따른 명란의 위생학적 특성" 한국수산과학회지 52(4):334-343 (2019).
- Kamani, J., Schaer, J., Umar, A. G., Pilarshimwi, J. Y., Bukar, L., González-Miguel, J., & Harrus, S. (2022). Molecular detection and genetic characterization of Anaplasma marginale and Anaplasma platys in cattle in Nigeria. Ticks and Tick-Borne Diseases, 13(4), 101955. https://doi.org/10.1016/j.ttbdis.2022.101955
- Gurpinar, S. S., Kart, D., & Eryilmaz, M. (2022). The effects of antidepressants fluoxetine, sertraline, and amitriptyline on the development of antibiotic resistance in Acinetobacter baumannii. Archives of Microbiology, 204(4). https://doi.org/10.1007/s00203-022-02853-6
- Baydar, E., Aydogdu, U., Utuk, A. E., Kaya, F., Timurkan, O., Erol, U., & Babur, C. (2022). Infectious diseases in rising riding-horse and donkey milk industry: First detection of Neospora sp. antibodies in donkeys in Turkey. https://doi.org/10.21203/rs.3.rs-1454386/v1
Controls and Related Product: