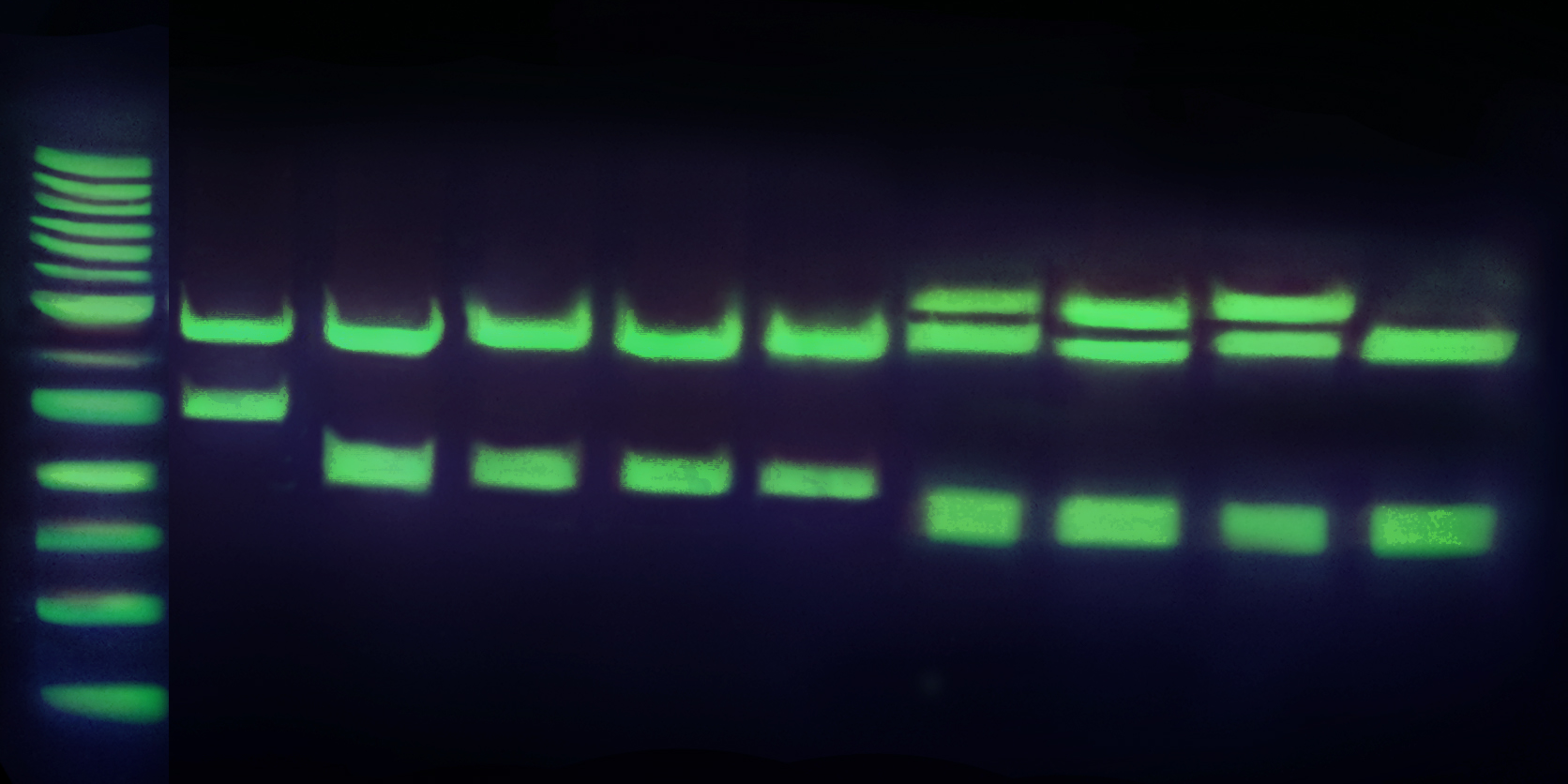
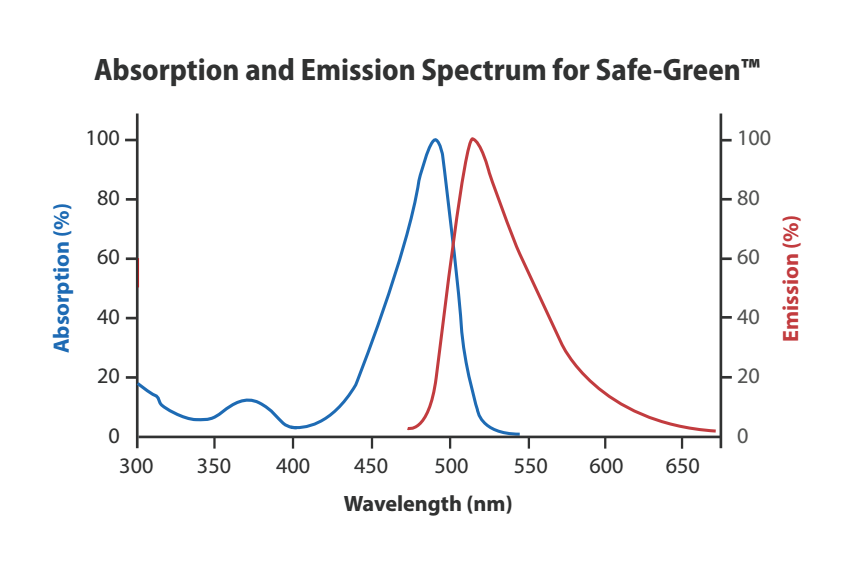
Safe-Green™
Specifications
| SKU | G108-G |
| Name | Safe-Green™ |
| Unit | 1.0 ml |
| Stain Color | Green |
| LED Viewer Compatibility | Yes |
| SafeView Series | Loading Dye |
| Description |
Safe-Green™ is a new and safe nucleic acid stain for the visualization of nucleic acids in agarose and polyacrylamide gels. This dye eliminates the need for toxic Ethidium Bromide (EtBr, a potent mutagen), commonly used in gel electrophoresis. Features:
|
| Applications |
Safe detection of dsDNA, ssDNA and RNA in agarose and polyacrylamide gels. |
| Storage Condition |
Store at 4°C for up to 2 years. |
| Shipping Conditions |
Shipped on blue ice packs. |
| Note |
All SafeView DNA Stains are ISO-13485 certified. Dispose of SafeView DNA Stains as you would any other non-carcinogenic fluorescent dye (eg. Acridine orange; Propidium iodide). |
FAQs
| Can SafeView Classic be a replacement for ethidium bromide? Can I do gel extraction with it? | |
|
SafeView Classic can be used as a complete replacement for your ethidium bromide workflow. Yes, SafeView Classic can be used during gel extraction - in house testing demonstrated that gel extraction of DNA fragments using a SafeView Classic gel resulted in no decrease in ligation efficiency vs ethidium bromide. |
| How does SafeView work and why is it not carcinogenic? | |
|
abm's SafeView products contain fluorescent compounds that have a strong affinity to DNA and RNA nucleic acids. Once bound to nucleic acids, the compound fluoresces under specific wavelength of light which can then be visualized using a standard UV/Blue light imager. There may be some unknown effects of SafeView products that have not been documented in literature but these would also apply to popular SYBRSafe as well; however, SafeView products are not as carcinogenic as ethidium bromide. |
| How do I use SafeView products? | |
|
Safe-Red and Safe-Green are in a 6X loading dye format: mix samples and DNA marker with Safe-Red or Safe-Green at a 1:5 (dye : sample) dilution ratio, and load onto an unstained gel. SafeView Classic and Safe-Red Gel are in a 10,000X format: for pre-cast, add 10µl SafeView Classic or Safe-Red Gel per 100 ml molten agarose, mix gently and cast the gel; for post-stain, prepare and run an unstained agarose gel, next submerge the gel in post-staining solution of 30 μl SafeView Classic or Safe-Red Gel per 100 ml 1X TAE or 1X TBE buffer for 30min in the dark with light agitation, image accordingly. |
| Can the SafeView products differentiate double stranded vs single stranded nucleic acids? | |
|
No, our SafeView products will bind to both double-stranded and single-stranded nucleic acids, albeit with lesser efficiency for single-stranded nucleic acid species.
|
| At what temperature do I store the SafeView products? | |
|
SafeView Classic, Safe-Red and Safe-Red Gel should be stored at 18-25°C. Safe-Green should be stored at 4°C. |
| Do I need a special filter for photography of DNA gels stained with SafeView products? | |
|
All SafeView products are compatible with UV or Blue Light imagers. However, Safe-Green and SafeView Classic are more optimally visualized under Blue Light, making these stains safer for the user and their samples.
|
| How long does SafeView Classic stain last in a gel? | |
|
Our in-house testing has shown that SafeView Classic stained gels (>10ng DNA loaded per lane) can still be effectively visualized up to 1 week later with only a slight decrease in brightness. Gels should be stored properly to maintain a good signal, sealed in a plastic bag with wet paper towel loosely wrapped around the gel, away from light. Similarly, a pre-cast unused SafeView Classic gel can be stored and used up to 1 week after being prepared provided they are stored in ideal conditions (sealed in plastic bag, away from light).
|
| What is the sensitivity of the SafeView products? | |
|
Safe-Green: 0.2-0.6 ng DNA per band Safe-Red: 0.6-1.0 ng DNA per band Safe-Red Gel: 0.6-1.0 ng DNA per band SafeView Classic: 1.0-2.0 ng DNA per band |
| Can SafeView products be used to post-stain gels? | |
|
Yes, SafeView Classic and Safe-Red Gel can both be used for either pre-cast or post-stain gels.
|
| Why is the EtBr signal stronger in the pictures when I compare SafeView with EtBr? | |
|
Most gel imaging systems have been optimized for EtBr so this is a likely reason why the EtBr signal may be stronger in the pictures.
|
| What is the functional pH range for Safe-Green? | |
|
Safe-Green works optimally between pH 7 to 9.
|
| Will I need an additional loading buffer for my samples? | |
|
If using Safe-Green or Safe-Red, you will not need to add any additional loading dyes or buffers. If using SafeView Classic or Safe-Red Gel, you will need to add an appropriate DNA loading dye to your samples in order for samples to properly settle and stay in the wells during electrophoresis. |
| We see migrations and band shifting of our fragments when using Safe-Green. Are there any recommendations that you can give us to minimize this band shifting? | |
|
Band shifting to a certain degree is unavoidable for any nucleic acid stain as most of these fluorescent compounds are large, positively charged molecules. Loading dye format nucleic acid stains, while convenient, may also suffer from more prominent band shifting due to the stain binding and migrating with the sample simultaneously during electrophoresis. Post-staining, while takes longer to complete, may be the optimal choice of nucleic acid stain strategy if band shifting issue is a priority. |
| I cannot see 100bp and 200bp bands on a 1% gel. What should I do? | |
|
It is often difficult to detect smaller 100bp and 200bp bands on a 1% gel especially if samples are of low concentration. We recommend visualizing smaller fragments on a higher concentration gel - ideally 2% agarose.
|
| Can I use SafeView products in polyacrylamide gels? | |
|
SafeView products are designed for use with agarose gels, and are not optimized for polyacrylamide gels.
|
| Which of the SafeView products will work with blue light / LED? | |
|
All of our SafeView products are compatible with UV and blue light.
|
| Does the dye migrate in a 1% agarose gel? | |
|
Yes, the dye should migrate on a 1% gel.
|
| What is the concentration of G108-G? | |
|
6X loading dye
|
| Would adding Safe-Green to the DNA ladder interfere with the migration rate of the loading dye of the ladder? | |
|
No, adding Safe-Green to the DNA ladder will not interfere with the DNA ladder's loading dye. You still need to add Safe-Green to the DNA ladder at the same ratio. To be more technically correct, Safe-Green, and all other EB alternative DNA binding dyes, alter the migration pattern of DNA on agarose gel by shifting DNA to a larger molecular size; it is just simply because the overall molecular size of a particular DNA band increases as more dye is binding onto it. This is why we recommend mixing the DNA ladder and Safe-Green at the exact same 1:6 ratio, so everything on the gel will be subjected to the same concentration of Safe-Green and thus having the same shift.
|
| What is the size of the tracking dye in Safe-Green after electrophoresis? | |
|
The tracking dye should be at ~300 bp after electrophoresis.
|
| Can Safe-Green penetrate the nucleus? | |
|
Yes, our Safe-Green™ stain indeed has the ability to penetrate the nucleus.
|
| What color is the tracking dye during electrophoresis? | |
|
Safe-Green and Safe-Red contain a blue tracking dye which will migrate at ~300bp after electrophoresis.
|
References
- Ibeagha-Awemu, EM et al. "Proteomics, genomics, and pathway analyses of Escherichia coli and Staphylococcus aureus infected milk whey reveal molecular pathways and networks involved in mastitis" J. Proteome Res. 9 (9):4604-4619 (2010). DOI: 10.1021/pr100336e. PubMed: 20704270. Application: PCR Products Viewing.
- Diaz-Balzac, CA et al. "Calbindin-D32k is localized to a subpopulation of neurons in the nervous system of the sea cucumber Holothuria glaberrima (Echinodermata)" PLoS ONE 7 (3):e32689 (2012). DOI: 10.1371/journal.pone.0032689. PubMed: 22412907. Application: PCR Products Viewing.
- Machida, RJ et al. "PCR primers for metazoan nuclear 18S and 28S ribosomal DNA sequences" PLoS ONE 7 (9):e46180 (2012). DOI: 10.1371/journal.pone.0046180. PubMed: 23049971. Application: PCR Products Viewing.
- Kim, NH et al. "Reactive oxygen species regulate context-dependent inhibition of NFAT5 target genes" Exp. Mol. Med. 45:e32 (2013). DOI: 10.1038/emm.2013.61.. PubMed: 23867654. Application: PCR Products Viewing.
- Goo, JS et al. "Bacillus thuringiensis: a specific gamma-cyclodextrin producer strain" Carbohydr. Res. 07-Dec:386 (2014). DOI: 10.1016/j.carres.2013.12.005. PubMed: 24456970. Application: PCR Products Viewing.
- Pyo, JS et al. "Activation of nuclear factor-κB contributes to growth and aggressiveness of papillary thyroid carcinoma" Pathol. Res. Pract. 209 (4):228-232 (2013). DOI: 10.1016/j.prp.2013.02.004. PubMed: 23528368. Application: PCR Products Viewing.
- Ish-Shalom, S et al. "Analysis of fungal gene expression by Real Time quantitative PCR" Methods Mol. Biol. 638:103-114 (2010). DOI: 10.1007/978-1-60761-611-5_7. PubMed: 20238263. Application: PCR Products Viewing.
- Lam, SW et al. "Rapid, specific and quantitative polymerase chain reaction (PCR) detection of pathogenic protozoa Entamoeba histolytica for drinking water supply" Water Science & Technology: Water Supply 11 (4):418-425 (2011). DOI: 10.2166/ws.2011.057. PubMed: 67657423. Application: PCR Products Viewing.
- Chang, KF et al. "First report of Fusarium proliferatum causing root rot in soybean (Glycine max L.) in Canada" Crop Prot 67:52-58 (2015). DOI: 10.1016/j.cropro.2014.09.020. Application: PCR Products Viewing.
- Mun, Y. S., & Hwang, Y. J. "Novel spa and Multi-Locus Sequence Types (MLST) of Staphylococcus Aureus Samples Isolated from Clinical Specimens in Korean" Antibiotics 8(4):202 (2019). DOI: 10.3390/antibiotics8040202.
Controls and Related Product: