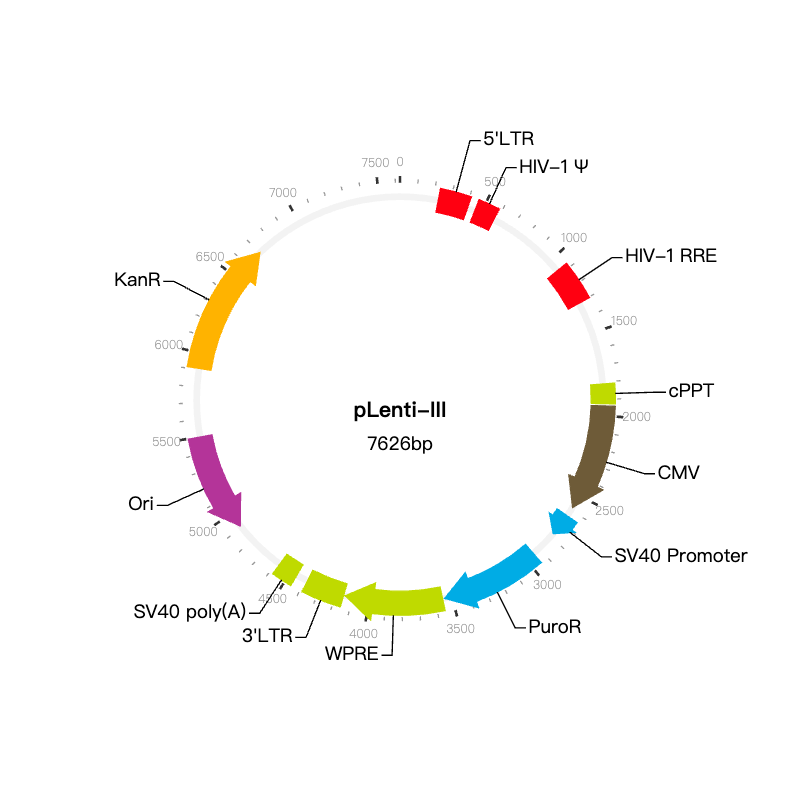
NEK4P3 Lentiviral Vector (Human)
Specifications
Print Custom Datasheet
| Accession Number | NM_001061.7 |
| Cat. No. | 46314061 |
| Gene Name | TBXAS1 |
| Full Gene Name | thromboxane A synthase 1 (platelet) |
| Species | Human |
| Alias | CYP5, CYP5A1, THAS, TXS, TXAS, TS |
| Alt. Accession Number | BC041157 , NM_001130966.5 |
| Full Gene Name | thromboxane A synthase 1 (platelet) |
| Insert Size | 1605 bp |
| Vector Backbone | pLenti-GIII-CMV (Custom Option available) |
Vector |
|
| Product Name | TBXAS1 Lentiviral Vector (Human) (CMV) (pLenti-GIII-CMV) |
| Unit | 1.0 µg DNA |
| Applications | Direct non-viral plasmid transfection for immediate expression. |
Virus |
|
| Product Name | TBXAS1 Lentivirus (Human) (CMV) (pLenti-GIII-CMV) |
| Unit | 1.0 µg , 2 x 200 µl |
| Titer | >107 IU/ml (Custom Option available) |
| Applications | Package into lentiviral particles for high efficiency transduction and stably integrated expression. |
Documents
Supporting Protocol
QC
FAQs
| What sites are compatible with your lentivirus backbone? What promotor(s) do your LV backbones have? What is the approx time frame to generate this type of construct? | |
|
We have a series of lentiviral vector with different promoters like EF1a, CMV, UbC, PGK. Please open the following links for site compatibility or send us your vector map. The subcloning can be done within 2 weeks.
http://www.abmgood.com/Viral-Expression-System/lentivirus-expression-systems.php?csn=14&ssn=3884
|
| I bought pLenti-III-promoterless-GFP vector and transfected into 293T cells and I found many cells express GFP. Is it normal? | |
|
Yes. GFP should not expressed if it is in a promoterless plasmid, however retroviral and lentiviral vectors all have 5'-UTR elements which naturally have some promoter function. When you subclone your own promoter between GFP and 5'-UTR, this function will be blocked by the promoter of your choice.
|
| How should I store my lentivirus? | |
|
Aliquots should be made for the lentivirus and stored at -70 degrees Celsius.
|
| What generation is your lentivirus? | |
|
It is third generation.
|
| What is our lentivirus based on? | |
|
Our system is based on inactivated HIV. All our packaging plasmids cannot be integrated into the host and they are transiently expressed only.
|
| What is the function of 3’ acceptor sites in lentiviral vectors? | |
|
It's for biosafety reason. The 5' splice donor and 3' acceptors sites enhance the biosafety of the vector by facilitating removal of the packaging sequence and RRE such that expression of the gene of interest in the transduced host cell is no longer Rev-dependent (Dull et al., 1998). It will inhibit viral production in stable cell lines.
|
| Is your lentiviral system Tat dependent or independent? | |
|
Tat-Independent.
|
| How do you ensure that your lentivirus is replication-defective? | |
|
Lentiviral vector stocks are tested for the presence of replication-competent lentivirus (RCL) by monitoring p24 antigen expression in the culture medium of transduced 293T cells for 30 days. Since the vectors are replication-defective, no amplification of the p24 expression would normally be expected. However, if there were an increase in P24 signal over time, it would be indicative of RCL contamination. The p24 expression is assayed by qPCR.
|
| How efficient is the lentivirus transduction? | |
|
This depends on the cell line or type as each one is different. In general, the lentivirus is very efficient in most cell lines with 100% for 293 and other commonly used cells. The lowest efficiency observed is 10% and customers have to do evaluation for a specific cell line using EGFP lentivirus. For customers that just need to generate a stable cell line, 1% trandsuction efficiency is enough as every transduced cell is permanently integrated with the vector. Therefore, 10ml of 10^6 cfu/ml would contain more than enough transduced cells.
|
| How do I increase viral titre in my target cells? | |
|
Usually, there is no need to increase viral titres for in-vitro experiments. In our company, we usually produce the virus in 293T cells and then infect the virus to the target cells. To increase the titre in the viral production, cell density should be around 80%. Packaging mix is also very important for viral production. Sometimes, lipid-based packaging mix does not work very well. You can try calcium phosphate transfection. If that also does not work, you can try our Lentifectin, which works very well in producing recombinant lentivirus. We use Lentifectin for our own custom services.
|
| What is the difference between the promoters? | |
|
EF1a promoter offers highest percentage of cells expressing transgene, follow by PGK and UBC. However, PGK promoter offers higher gene expression level in positive cells.
CMV: Immediate-early Cytomegalovirus virus promoter for high-level expression in a wide variety of mammalian cell lines
EF1-1a: Human elongation factor 1a-subunit promoter for high-level expression
UbC: Human ubiquitin C promoter for high-level expression that is equivalent across a broad range of species and tissue types
SV40: Simian virus 40 promoter for high-level expression. Permits replication in cell lines expressing the large T antigen
PGK: Murine Phosphoglycerate Kinase-1 promoter for long-term persistent expression in cells that are susceptible to promoter silencing from methylation or histone deacetylation , such as undifferentiated embryonic stem (ES) cells.
|
| Does your system work with our packaging mix? | |
|
This will depend on your system. It may or may not work. Ours has been tested to be compatible with OpenBiosystems and Invitrogen but we have not tested with others suppliers.
|
| How come after infection, the target cells’ life spans are shorter than the negative control (target cells without the lentivirus)? | |
|
For the infection process, the supernatant is used to infect the target cells. The supernatant was collected from the viral production stage and based on DMEM media that have been depleted of nutrients at the point of harvest. These characteristics of the supernatant may create a stressful environment for the target cells. DMEM may not be appropriate for the target cells and/or the depleted media is not enough to maintain the target cells. Furthermore, the lentiviral components make the cells more sensitive to cell death.
|
| Does MOI affect antibiotic resistance? | |
|
Higher MOI will provide more copies of the antibiotic resistance gene per cell. Cells containing multiple copies of the resistance gene can withstand higher antibiotic concentrations than those at lower MOIs. The concentration of antibiotic should be adjusted to a level that will select for the population of transduced cells you wish to select for, without going below the minimum antibiotic concentration you have established in your killing curve.
|
| What is the difference between Retro-, Lenti-, and Adeno- viruses? | |
|
Retrovirus: Classic, can integrate into the genome but with low transduction efficiency. They are useful for gene transfer and protein expression in cells that have low transfection efficiency with other transfection reagents. Lentivirus: Can integrate into the genome with relatively high transduction efficiency and they are very useful for cells that have low transfection efficiency with other transfection reagents. No special competent cells required, as they are stable plasmids. Lentiviruses are a powerful tool for stable gene transfer to both dividing and non-dividing cells in vitro and in vivo. Adenovirus: Only work transiently (about 7 days) but have almost 100% transduction efficiency. Adenoviruses can infect a broad range of cell types with the highest efficiency and infection is not dependent on active host cell division. A second key feature is that high virus titers and high-level gene expression can be obtained in most mammalian cells.
|
| What are the correct concentration units for each recombinant viral particle? | |
|
For lentiviruses and retroviruses, they are measured in CFU/ml (colony-forming units per millilitre). Transduction with lentiviruses and retroviruses can cause the formation of colonies, which can be quantified for concentration. For AAV the titer is measured as genome copies per mL (GC/mL). Adenoviruses are measured as PFU/ml (plaque-forming units per millilitre). Transduction with adenoviruses will kill packaging cells, forming plaques in the process for quantification. The concentration for all three types of viruses can also be classified as IU/ml (Infectious Units/ml). Ultimately, the units refers to the viral particles and different units reflect the different assays involved.
|
| For a lentiviral vector with a kanamycin resistance gene, what concentration of kanamycin should be used? | |
|
We recommend 50ug/ml.
|
| How do I calculate the MOI? | |
|
MOI = Product Titer (IU/ml) x Virus Volume (ml) / Total Cell Number
|
| What do I use to check if my cells were successfully immortalized by the SV40 agent? | |
|
We have an SV40 T antibody that can be used for the western blot analysis. The catalog number is G202.
Otherwise, a qPCR primer can be designed on the SV40 gene for qPCR analysis. The sequence can be found in the link below:
http://www.abmgood.com/pLenti%20SV40-Vector-Location-Map.html
|
| What are the primers to use for SV40 identification? | |
|
SV40 Forward Primer Sequence
5’ ACTGAGGGGCCTGAAATGA
SV40 Reverse Primer Sequence
5’ GACTCAGGGCATGAAACAGG
These are qPCR primers and the band size is 61 bp.
|
| Why is my lentiviral infection of lower efficiency than transduction? | |
|
There are many factors including insert size, insert sequence (specific sequence), and bacteria strain used for viral vector production, that can affect lentivirus packaging titer. No on can predict for sure which inserts will work better. Transduction can have higher transduction efficiency and higher level of gene expression as over 100 copies of DNA can get into cells. But only about 1% transduced cells integrated into host cells. Lentivirus efficiency can often be lower than transfection and expression level are also lower than transfection expression due to only 1-10 copies of viral particles getting into cells. In addition, the efficiency is also cell line dependent. We have documented well that lentiviruses can easily transduce 293 and MDA-MB-468 cells. Please test those two cell lines to be sure whether it is a titer issue or perhaps that this particular cell line is difficult for lentivirus to transduce.
|
| What starting range of puromycin should I use for the antibiotic selection? | |
|
The concentration must be optimized case-by-case and is cell type dependent. On average, typical selection amounts are around 0.1 to 0.5 ug per ml.
|
| Is our system compatible with psPAX2 and pDM2.G? | |
|
Yes, it is.
|
| I cloned in my promoter into the Promoterless GFP lenviral vector, packaged the virus, and transduced my cells. I did not see any GFP expression in my cells. Any suggestions? | |
|
This indicate that the promoter is very weak, which allows detection of your luciferase, but not GFP. Here are some optimization tips:
1. Do transfection in 293T cells to see whether there is proper GFP expression. Add an inducer if the promoter inducer is known.
2. Do a high titer lentivirus production and transduce your own cells using as high of the titer as possible.
3. Select for clones with puromycin. Weak signals should be seen from some of the clones after the expression of GFP has accumulated in the cells. If not, a WB analysis of GFP can also prove that GFP is expressed.
|
| What is the vector design for LV011-a Lenti-GFP? | |
|
The GFP gene is cloned via the KpnI and XhoI cut sites into the LV009 - Lenti-Easy-His Tag Vector without the His Tag.
|
| Which are the excitation and emission wavelengths of the RFP protein expressed by your lentiviral vector? | |
|
The maximum excitation wavelength is 588nm and the maximum emission wavelength is 633 nm for the RFP.
|
| For the pLenti-GIII-CMV-GFP-2A-Puro, are there recombinant sites flanking the insert? | |
|
Yes, there is an extra ~125bp to either side of the CDS insert. Thus, if EcoRV is used, the band will be the insert size plus 250bp.
|
| For the GIII third generation lentiviral vectors, are there recombination sites flanking the insert sequence? | |
|
Yes, there is an extra ~125bp to either side of the CDS insert. Thus, if EcoRV is used, the band will be the insert size plus 250bp.
|
| We want to study promoter function. If the LTR is leaky, would it interfere with the our promoter? | |
|
If your promoter is over 500bp, it is less likely to see any effect from the LTR. It is promoter dependent.
|
| Are they high copy or low copy plasmids? What is expected plasmid DNA yield? | |
|
These are high copy plasmids and should be propagated with an authentic DH5a strain. We typically see a yield of 300-500ug DNA from a 250mL culture.
|
| I see that some of your vectors contain a tetO reg site. Does that mean that their expression can be inhibited by the tet repressor? | |
|
The TetO reg site is present in our lentivectors to facilitate packaging of toxic genes into lentiviruses if required. If your transfected cells express the Tet repressor, the TetO reg site will be activated.
If there is no Tet repressor, the promoter will function as normal. If you are using a cell line that is expressing Tet repressor, then you will require Dox to express the promoter.
|
| I see from the map that there is an HA tag after the GFP fusion vector. Is this tag expressed? | |
|
Yes this tag is expressed since there is no stop codon between the GFP and the tag. The stop codon is located at the end of the tag.
|
| What version is the luciferase for cat#LV092? | |
|
Firefly luciferase.
|
| What is your HA tag sequence? | |
|
5'-tacccatacgacgtcccagactacgct-3'
|
| Do you have viral particles for this product? | |
|
We offer custom lentivirus packaging services for a range of viral titers. Please go to the following link for more details:
http://www.abmgood.com/Custom-Lentivirus-Subcloning-Services.html
|
| What species is the PGK promoter from? | |
|
Mouse Genome Accession number: NC_000086.7
|
| Which strain of competent E.coli do you recommend for amplifying this vector? | |
|
We recommend amplifying all of our pLenti vectors in DH5α competent cells
|
| What advantages / disadvantages exist between the Lenti-SV40, -SV40T, and SV40T+t vectors? | |
|
There are simply differences in the content of all vectors due to customer demand for variety. Lenti-SV40 will contain the whole SV40 gene, -SV40T, the large T Antigen only, and -SV40T&t the large and small T antigens only.
It is up to the end user to decide which vectors will best suit their project, however we have successfully used Lenti-SV40 (whole gene) in a wide range of immortalization projects.
|
| Are the 293T cells that come along with LV060 Kit resistant to Geneticin? | |
|
The 293T cells we provide do not have G418 resistance.
|
| What is the difference between the pLenti-III and the pLenti-GIII vectors? | |
|
The difference is only in the availability of cloning sites for insertion of the gene.
|
| Are the vectors Lenti Promoterless GFP (LV060) and Lenti GFP Control vector (LV011a) high copy no. or low copy no. plasmids? | |
|
All of our pLenti vectors are high copy no. plasmids
|
| I also need an empty vectors as a control, how do I go about ordering this? | |
|
Please see our listings of available blank control vectors: http://www.abmgood.com/Lentiviral-Blank-Control-Vectors.html
We can also produce custom control vectors upon request, please email technical@abmgood.com with specific inquiries
|
| Do your pLenti vectors include a chimeric RSV promoter upstream of the 5' LTR? | |
|
Yes, our pLenti vectors do include this RSV chimeric promoter sequence
|
| While cloning into LV059, do we have to put our promoter and gene should be in-frame with the GFP's ATG? | |
|
If you are just inserting a promoter, this does not need to be in frame.
However, if you are inserting a promoter AND a gene, then yes, it will need to be in frame with the ATG of the GFP.
|
| How are your DNA pLenti vectors supplied? | |
|
DNA is supplied in 10mM Tris (unless otherwise requested) and intended for direct transformation in DH5alpha cells.
|
| What is the accession number for the SV40? | |
|
The SV40 covers the entire genome and the accession number is J02400.1. You can use this information to design primers for conventional PCR as well.
|
| How do I calculate MOI? | |
|
MOI refers to the number of viral particles per cell used in your infection, e.g. an MOI of 5 indicates that there are five infectious units (IU) or transducing units (TU) for every cell in the well. To determine the multiplicity of infection (MOI), calculate the number of viral particles added per well then divide this number by the number of cells you have seeded into the well.
We would also recommend transducing with a range of MOIs as different cell types may require different MOIs for successful transduction.
MOI = Virus titer (IU/ml) x Virus Volume (ml) / Total cell number
|
| Which packaging system should I use, 2nd or 3rd generation? | |
|
All of abm's lentviral vectors are compatible with 2nd and 3rd generation packaging systems LV003 and LV053 available from abm. Please note, only packaging mixes produced by abm have been tested in house and therefore carry our guarantee for high titer virus production. If it is desirable to use other packaging plasmids obtained from a different source, the compatibility must be tested and determined by the end user.
|
| Can you please advice on a protocol for vector extraction from filter paper? | |
|
1) Cut out the circle on the filter paper (this is where the DNA will be spotted), and then cut it into tiny pieces into a 1.5ml tube.
2) Put 30-50ul of PCR-grade water, 10mM Tris, or TE buffer to elute the DNA from the filter paper. The liquid may be completely soaked up by the filter, this is OK. Cap the tube and leave at room temperature for 5 minutes.
3) Centrifuge the tube at high speed for 1 minute to collect the liquid, or use a pipette and "compress" the filter paper at the bottom of the tube to squeeze out as much liquid as possible. Collect the liquid in a new tube, and then use DH5a competent cells to amplify the plasmid, use the suitable antibiotic to select for positive clones.
|
| How long after transduction can the infection efficiency be observed? | |
|
You can observe transduction efficiency from 48 hours up to 5 days after infection.
|
| Where is the polyA signal in your vectors? | |
|
An SV40 poly A signal is located at the end of the 3'LTR. It is designed this way to ensure a high amount of transcriptional RNA is present so that a high viral titer is obtained. Having a polyA signal upstream of the 3'LTR can cause premature RNA transcription and reduce the amount of transductionally active RNA. Lentivirus is an RNA virus and having a polyA signal may affect viral packaging, thus it is removed from the expression cassette and placed at the end of the 3'LTR.
We have received positive feedback from previous customers in regards to the high and stable expression levels of our lentivectors.
|
| Regarding LV022, why is a poly-A terminator not found after the HA-tag? | |
|
The SV40 polyA terminator in the 3'LTR will be sufficient to signal for polyadenylation of the insert transcript. We refrained from putting a polyA terminator after the HA-tag as it could lower the number of tranductionally active RNA transcripts and so will lower the titre of viruses produced.
|
| What is the volume supplied? | |
|
Normally the concentration of our vectors are at 0.1ug/ul so there should be around 10ul in terms of volume.
|
| Are design primers with a Kozak sequence required or is ATG enough? | |
|
Yes, a Kozak sequence immediately upstream of the ATG is required to ensure proper protein translation. This is true for all mammalian expression vectors that we offer.
Please note that blank vector/viruses do not express anything so the kozak sequence will be absent.
|
| What are the primers to use for SV40T and SV40T tsA58 detection? | |
|
PCR primers:
SV40T Forward Primer Sequence
5’ AGCCTGTAGAACCAAACATT 3'
SV40T Reverse Primer Sequence
5’ CTGCTGACTCTCAACATTCT 3'
The two primers should amplify the region between 3677-4468bp, giving a 792bp fragment.
|
| Regarding Lenti-III-UbC Vector Cat# G300, is a Kozac consensus sequence necessary upstream of the insert's start codon? | |
|
Yes, it is necessary to have a Kozak sequence immediately upstream of the insert's start codon. We normally use the sequence "GCCACC".
|
| Regarding Lenti-III-UbC Vector Cat# G300, is a polyA tail to be added? | |
|
A polyadenlyation site is located in the 3'LTR of the Cat# G300 vector. This will be sufficient to signal for the adding of the polyA tail to the mRNA transcript.
|
| What is the sequence of the SV40 large T antigen? | |
|
This information can be accessed on this page by clicking on "pLenti-SV40-T" under vector map. The Large T antigen is at position 5079-5927.
|
| Which lentiviral genes were removed from the viral vector to render it replication deficient? | |
|
The gag, pol, rev, env, vif, vpr, tat, vpu, and nef elements involved in lentiviral replication are not present in our lentiviral systems.
|
| Where is the cDNA terminator sequence for Cat# LV009? | |
|
This vector has a His tag at the end of the MCS. There are 2 STOP codons that follows immediately after the His tag. If you don't want the His tag to be expressed, you may clone in your GOI with the STOP codon in front of the His tag.
|
| Does your lentiviral system contain the WPRE gene? If so, is this the wildtype WPRE sequence? | |
|
The WPRE section is present in our lentiviral system and it is the standard wildtype version. You may align the wildtype sequence against our listed vector sequence.
|
| For G221 and LV620, what does the 'V12' in RasV12 mean? | |
|
The V12 means that amino acid # 12 is mutated from a Valine to a Glycine. Other than that, the sequence matches the coding region of HRAS perfectly (NM_005343).
|
| Where is the SV40T tsA58 gene sequence? | |
|
The SV40T tsA58 gene is located between 3138-5264bp, with the Alanine-to-Valine mutation at amino acid 438.
|
References
- Huang, S et al. "Coupling of small leucine-rich proteoglycans to hypoxic survival of a progenitor cell-like subpopulation in Rhesus Macaque intervertebral disc" Biomaterials 34 :6548-6558 (2013). DOI: 10.1016/j.biomaterials.2013.05.027. Application: Gene Expression.
- Neacsu, O et al. "IGF-I attenuates FFA-induced activation of JNK1 phosphorylation and TNFα expression in human subcutaneous preadipocytes" Obesity 21:1843–1849 (2013). DOI: 10.1002/oby.20329. Application: Lentiviral Expression.
- Laryea, G et al. "Site-specific modulation of brain glucocorticoid receptor and corticotropin-releasing hormone expression using lentiviral vectors" Mol. Cell. Endocrinol. 371:160-5 (2013). DOI: 10.1016/j.mce.2012.12.005. PubMed: 23261985. Application: Gene Expression.
- Chan, M et al. "Development and application of honey bee in vitro systems" Thesis : (2012). Application: Gene Expression.
- Thorn, M et al. "Liver metastases induce reversible hepatic B cell dysfunction mediated by Gr-1+CD11b+ myeloid cells" J Leukoc Biol 96(4):883-894 (2014). DOI: 10.1189/jlb.3A0114-012RR. PubMed: 25085111.
- Li, F et al. "Second messenger role for Mg2+ revealed by human T-cell immunodeficiency" Nature 475:471-476 (2011). DOI: 10.1038/nature10246. Application: Bicistronic lentivector.
- Laryea, G et al. "Site-specific modulation of brain glucocorticoid receptor and corticotropin-releasing hormone expression using lentiviral vectors" Mol Cell Endocrinol 371(1-2):160-5 (2013). DOI: 23261985. PubMed: 10.1016/j.mce.2012.12.005. Application: EF1a lentivector.
- Zhou, Y et al. "Genetic Modification of Primate Amniotic Fluid-Derived Stem Cells Produces Pancreatic Progenitor Cells in vitro" Cells Tissues Organs 197(4):269-82 (2013). DOI: 23306211. PubMed: 10.1159/000345816. Application: Tricistronic lentivector.
- Chan, YC et al. "Downregulation of endothelial microRNA-200b supports cutaneous wound angiogenesis by desilencing GATA binding protein 2 and vascular endothelial growth factor receptor 2" Arterioscler Thromb Vasc Biol 32(6):1372-8 (2012). DOI: 10.1161/ATVBAHA.112.248583. PubMed: 22499991. Application: miRNA overexpression lentivector, gene overexpression lentivirus.
- Wieczorek, L et al. "Absence of Ca2+-stimulated adenylyl cyclases leads to reduced synaptic plasticity and impaired experience-dependent fear memory" Transl Psychiatry 2:e12 (2012). DOI: 10.1038/tp.2012.50. PubMed: 22832970. Application: Overexpression lentivector.
- Su, RJ et al. "Few Single Nucleotide Variations in Exomes of Human Cord Blood Induced Pluripotent Stem Cells" PLOS ONE 8(4):e59908 (201). DOI: 10.1371/journal.pone.0059908. PubMed: 23573220. Application: Overexpression lentivector.
- Wang, J et al. "CXCR6 Induces Prostate Cancer Progression by the AKT/Mammalian Target of Rapamycin Signaling Pathway" Cancer Res 68(24):10367-76 (2008). DOI: 10.1158/0008-5472.CAN-08-2780. PubMed: 19074906. Application: siRNA lentivector.
- Ou, Y et al. "Targeting of CRMP-2 to the Primary Cilium Is Modulated by GSK-3β" PLoS One 7(11):e48773 (2012). DOI: 10.1371/journal.pone.0048773. PubMed: 23185275. Application: siRNA lentivector.
- Wen, Z et al. "MicroRNA-377 Regulates Mesenchymal Stem Cell-Induced Angiogenesis in Ischemic Hearts by Targeting V" PLoS One 9(9):e104666 (2014). DOI: 10.1371/journal.pone.0104666. PubMed: 25251394. Application: Inhibitor miRNA lentivector.
- Ruppender, N et al. "Cellular Adhesion Promotes Prostate Cancer Cells Escape from Dormancy" PLoS One 10(6):e0130565 (2015). DOI: 10.1371/journal.pone.0130565. PubMed: 26090669.
- Song, J et al. "A long non-coding RNA, GAS5, plays a critical role in the regulation of miR-21 during osteoarthritis" J Orthop Res 32(12):1628-1635 (2015). DOI: 10.1002/jor.22718. PubMed: 25196583. Application: Gene Delivery, Lentivirus.
- Choe, C et al. "Crosstalk with cancer-associated fibroblasts induces resistance of non-small cell lung cancer cells to epidermal growth factor receptor tyrosine kinase inhibition" OncoTargets and Therapy 2015.8:3665-3678 (2015). DOI: 10.2147/OTT.S89659. Application: Stable Expression.
- Wang, J et al. "Radiofrequency hyperthermia-enhanced herpes simplex virus-thymidine kinase/ganciclovir direct intratumoral gene therapy of hepatocellular carcinoma" American Journal of Cancer Research 6.9:2054–2063 (2016). DOI: 10.1080/02656736.2016.1229045. Application: Animal infection.
- Fukumoto, T., Zhu, H., Nacarelli, T., Karakashev, S., Fatkhutdinov, N., Wu, S., ... & Zhang, L. "N6-methylation of Adenosine of FZD10 mRNA Contributes to PARP Inhibitor Resistance" Cancer research 79(11):2812-2820 (2019).
- Holdsworth-Carson, S. J., Colgrave, E. M., Donoghue, J. F., Fung, J. N., Churchill, M. L., Mortlock, S., … Rogers, P. A. W. "Generation of immortalized human endometrial stromal cell lines with different endometriosis risk genotypes" MHR: Basic Science of Reproductive Medicine 25(4):194–205 (2019). DOI: 10.1093/molehr/gaz006.
- Hsieh, M.-C., Peng, H.-Y., Ho, Y.-C., Lai, C.-Y., Cheng, J.-K., Chen, G.-D., & Lin, T.-B. "Transcription Repressor Hes1 Contributes to Neuropathic Pain Development by Modifying CDK9/RNAPII-Dependent Spinal mGluR5 Transcription" International Journal of Molecular Sciences 20(17):4177 (2019). DOI: 10.3390/ijms20174177.
- Huang, D., Tang, L., Yang, F., Jin, J., & Guan, X. "PIK3CA mutations contribute to fulvestrant resistance in ER-positive breast cancer" American Journal of Translational Research 11(9):6055 (2019).
- Jin, Y., Li, C., Xu, D., Zhu, J., Wei, S., Zhong, A., ... & Xia, Q. "Jagged1-mediated myeloid Notch1 signaling activates HSF1/Snail and controls NLRP3 inflammasome activation in liver inflammatory injury" Cellular & Molecular Immunology 1-12: (2019).
- Joshi, S., Wollenzien, H., Leclerc, E., & Jarajapu, Y. P. "Hypoxic regulation of angiotensin‐converting enzyme 2 and Mas receptor in human CD34 cells" Journal of Cellular Physiology 234(11):20420–20431 (2019). DOI: 10.1002/jcp.28643.
- Podgórska, E.. "Wybrane aspekty działania witaminy D3 jako czynnika wspomagającego leczenie czerniaków (Doctoral dissertation)" : (2019).
- Wang, J., Zuo, J., Wang, M. D., Xie, W. F., Bai, X. B., & Ma, X. D. "Receptor tyrosine kinase AXL is correlated with poor prognosis and induces temozolomide resistance in glioblastoma" CNS Neuroscience & Therapeutics. : (2019).
- Wang, J., Zuo, J., Wang, M., Ma, X., Gao, K., Bai, X., … Liu, H. "Polo‑like kinase�4 promotes tumorigenesis and induces resistance to radiotherapy in glioblastoma" Oncology Reports. : (2019). DOI: 10.3892/or.2019.7012.
Other NEK4P3 Products
NEK4P3
Human
NEK4P3
Lentiviral
Vector
NEK4P3
AAV
Vector
NEK4P3
Adenovirus
Vector
NEK4P3
Protein
Vector
NEK4P3
ORF
Vector
NEK4P3
siRNA
Vector
NEK4P3
miRNA
Vector
NEK4P3
3'UTR
Vector
NEK4P3
CRISPR Knockout
Vector
NEK4P3
CRISPR Activation
Vector
NEK4P3
Control Vectors & Viruses
Vector
NEK4P3
circRNA
Vector
NEK4P3
Internal
Vector